Dermal Oncoimmunology Junior Group
Dr. Hafsa Munir
Manipulating cancer-associated fibroblasts to favour a robust anti-tumor immune response
OUR RESEARCH
Why it matters
While huge leaps have been made to improve the detection, prevention, and treatment of skin cancer, the overall incidence has increased over the past 10 years, which is due in part to the climate crisis. Cutaneous melanoma comprises only 2% of all skin cancers but contributes to more than 80% of skin cancer-related deaths. Despite the success of immune checkpoint inhibitors for treating melanoma, 40-70% of patients develop resistance. Stromal cells in the skin can induce resistance to multiple cancer therapies and therefore are an attractive target for cancer therapy. The Dermal Oncoimmunology Lab aims to identify stromal mediated mechanisms of resistance and devise ways of modifying the state of the stromal cells to overcome therapeutic resistance.
MUNIR LAB
Lab focus
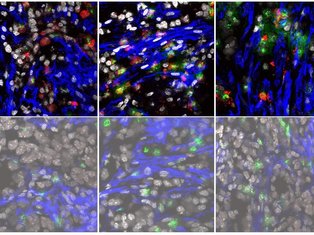
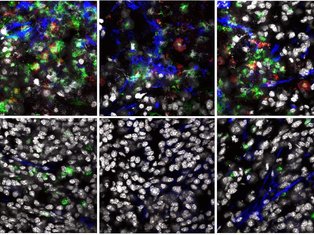
Cancers are a highly heterogenous group of diseases. This diversity is not restricted to the malignant cells, but rather extends to the surrounding microenvironment (ME; containing fibroblasts, endothelial cells and immune cells). Recent work has shown that the state of the tumour ME plays a significant role on the anti-tumour immune response and sensitivity to immune-checkpoint inhibitors.
While autoimmune disease and cancer are opposing conditions, both are indisputably interconnected. Indeed, both are chronic inflammatory conditions in which the ME regulates disease progression through effects exerted on immune cells. Additionally, the advent of therapeutic agents targeting immune checkpoints and that autoimmunity in cancer is a positive prognostic marker highlight how inducing an autoimmune-like state within tumours could be pivotal for developing novel ways to treat cancer.
Cancer-associated fibroblasts (CAFs) provide an attractive target for driving an autoimmune-like state in tumours as these cells are critical regulators of the immune response. In the Dermal Oncoimmunology lab, we place fibroblasts at the centre of immune-regulatory networks in the skin during autoimmunity and cancer. We strive to elucidate and compare the phenotype and function of subsets of skin CAFs with those from autoimmune skin lesions. In turn, we will manipulate the CAFs to mimic aspects of fibroblasts from autoimmune skin lesions to favour a robust anti-tumour immune response.
Our lab will combine multiplexed flow cytometry, confocal microscopy with single-cell sequencing and spatial transcriptomics to characterise the phenotype and function of fibroblasts and infiltrating immune cells in the skin of patients with cancer or autoimmune skin lesions. We will also use advanced imaging tools to assess tissue topography and location of fibroblast subsets in these conditions.
We will develop and utilise microfluidic platforms incorporating complex3D multicellular matrices, organotypic cultures and novel in vitro approaches to dissect the fibroblast-immune interactions. We will use genetic manipulation studies within these systems alongside transgenic mouse models to manipulate CAFs towards an autoimmune-like state and assess how this influences tumour growth and the immune landscape within the tumour.
Junior Group Leader Dermal Oncoimmunology
Dr. Hafsa Munir
 D. Jung
D. Jung
[since 2023] Junior Group Leader HI-TRON Mainz
[2021 – 2023] Postdoctoral Fellow at the Hospital for Special Surgery/Weill Cornell Medicine, USA
[2016 – 2021] Postdoctoral Fellow at the MRC Cancer Unit at the University of Cambridge, UK
[2012 – 2016] PhD at the Institute for Biomedical Research at the University of Birmingham, UK
[2019] Best talk prize at the annual Trinity College Forum, University of Cambridge
[2018] Best talk prize at the annual MRC Cancer Unit/Hutchison retreat
[2014] 26th UK Adhesion Society Meeting best poster prize
[2014] Travel Award, University of Birmingham
HI-TRON Mainz DKFZ
AG Munir
Obere Zahlbacherstr. 63
Building 911
55131 Mainz
Team
Dr. Micaela Domingues
Postdoc
Dr. Manuela Ecker
Postdoc
Shanice Gundel
PhD student
Dr. Alisa Lepper
Clinician Scientist
Hannah Nolan
Technician
Selected publications
- Mazzaglia C, Munir H, Huang YYS, Shields JD. Modelling structural and cellular elements and functional responses to lymphatic-delivered cues in a lymph node on a chip. Advanced Healthcare Materials. 2024; e2303720.
- Zimarino C, Moody W, Davidson S, Munir H*, Shields JD*. Disruption of CD47-SIRPα signaling restores inflammatory function in tumor-associated myeloid-derived suppressor cells. iScience. 2024; 27(4): 109546. (*co-corresponding author)
- Munir H, Jones JO, Janowitz T , Martins CP, Shields JD. Stromal-driven and Amyloid β-dependent induction of neutrophil extracellular traps modulates tumour growth. Nature Communications. 2021; 12(1):683
Lab News
01/2025: A new member joins the Munir lab: Alisa Lepper
01/2025: Two new members join the Munir lab: Micaela Domingues and Manuela Ecker
03/2025: A new member joins the Munir lab: Shanice Gundel